Web Designing
Expertise Overview
Software Application Development
Software Product Development
2/3 Tier Architecture
Multi Technology Services
Report for Mobile Devices
USFDA CFR11 Compliance
Audit Trail & Reports
Hardware Integration
Application Protection
SOP building
Application Validation
BCP & DRM Documentation
Product Areas
Clinical Research is a critical step in the evr growing Life Science arena. Clinical Research has a pivotal role to play in
New Drug discovery. Inforcom Technologies team holds deep domain knowledge about CRO – Contact Research Organisations
operations. The life cycle of a Clinical Research study / trial requires the understanding of numerous integrated processes.

 These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
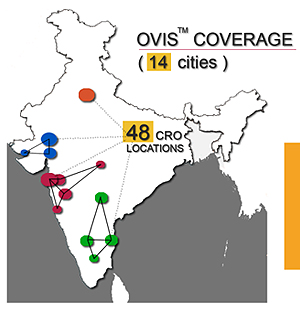
 OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
 Inforcom also offers
Inforcom also offers

 These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
Inforcom has two main product in the market :
VPMS - Volunteers and Project Management System VPMS is inhouse CRO application for confidential information storage. This intranet application offers modules for volunteers demographics and personal information. Further the details of screening and dosing with ADR information can also be saved. OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
 Inforcom also offers
Inforcom also offers
- CRO Project Management
- Blood Sampling and Sorting Labeling etc.
Clinical Research is a critical step in the evr growing Life Science arena. Clinical Research has a pivotal role to play in
New Drug discovery. Inforcom Technologies team holds deep domain knowledge about CRO – Contact Research Organisations
operations. The life cycle of a Clinical Research study / trial requires the understanding of numerous integrated processes.

 These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
 OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
 Inforcom also offers
Inforcom also offers

 These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
Inforcom has two main product in the market :
VPMS - Volunteers and Project Management System VPMS is inhouse CRO application for confidential information storage. This intranet application offers modules for volunteers demographics and personal information. Further the details of screening and dosing with ADR information can also be saved. OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
 Inforcom also offers
Inforcom also offers
- CRO Project Management
- Blood Sampling and Sorting Labeling etc.
Clinical Research is a critical step in the evr growing Life Science arena. Clinical Research has a pivotal role to play in
New Drug discovery. Inforcom Technologies team holds deep domain knowledge about CRO – Contact Research Organisations
operations. The life cycle of a Clinical Research study / trial requires the understanding of numerous integrated processes.

 These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
 OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
 Inforcom also offers
Inforcom also offers

 These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
These are controlled by humans as well as machines at various levels. Standard compliance is required at all stages which
also need to be implemented in Software Applications. Further these applications need to be validated as per the standards
of USFDA CFR – 11/2.
Inforcom Technologies offer their expertise and knowledge of CRO processes from screening work till to the PI tasks and
further to the management. Inforcom offer CRO software products as well as development services to create cusotmised software
applications.
Inforcom has two main product in the market :
VPMS - Volunteers and Project Management System VPMS is inhouse CRO application for confidential information storage. This intranet application offers modules for volunteers demographics and personal information. Further the details of screening and dosing with ADR information can also be saved. OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
OVIS - Online Volunteers Information System
OVIS is a cloud based service to track volunteers cross participation. This application is biometrics driven for accurate
identification. This services has become nation wide, to integrate CROs of India.
 Inforcom also offers
Inforcom also offers
- CRO Project Management
- Blood Sampling and Sorting Labeling etc.
Copyrights@2014.






