Web Designing
Expertise Overview
Software Application Development
Software Product Development
2/3 Tier Architecture
Multi Technology Services
Report for Mobile Devices
USFDA CFR11 Compliance
Audit Trail & Reports
Hardware Integration
Application Protection
SOP building
Application Validation
BCP & DRM Documentation
Product Areas
Online Volunteers Information System - OVISTM
Web based System for Clinical Trial Organisations to track Volunteers Cross Participation(Conceptualised and developed by Inforcom Technologies)
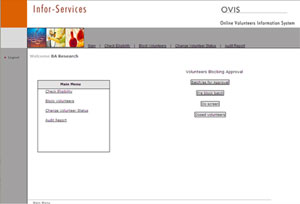
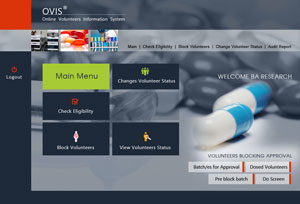
THE ISSUE :
Contract Research Organisations (CRO) or Clinical Trial based Organisations enroll volunteers for studies. These volunteers undergo screening and further the dosing cycles. At the end of the project, they are advised not to re-participate in such project for 3 months or other specified. This time period is to ensure sufficient blood regeneration and drug wash-out. Volunteers ignore this re-eligibility locking days and get enrolled at other such organisations to earn more. This cross participation can be a health hazard to the volunteer as well a potential case of erroneous results in the studies. Inforcom Technologies is an IT consulting firm with software services and products in the field of Life Science and other. Inforcom is offering a web based application service to CROs to track such participants who hop from one CRO to another.Activities at a CRO
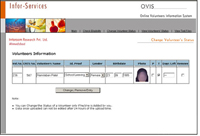
Volunteers approach CROs and enroll themselves for a clinical trail project. The CROs collect data of the volunteers in 'Volunteers & Project Management System - VPMS' (a clinical trial management application developed by Inforcom Technologies) or any other such tools. The participating CROs require to upload certain sharable data to OVIS - the web based system developed by Inforcom Technologies. Along with this data the lock-in period is also stored on the web. This data is the information for other Organisations to cross check the volunteers with.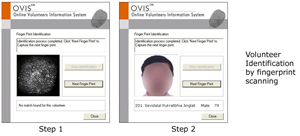
The Main Features of OVIS
- 24X7 access on Internet
- Biometrix Matching
- On the spot eligibility results
- Backup check by name and photo
- Individual statistical reports
- Audit trails for work review & monitoring
- Data edit locks against tempering
- Auto Check feature for quick file processing
- Password Protection
- Validated
The Eligibility check on OVIS
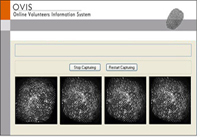
As a volunteer re-approaches a CRO for screening or dosing, the organisation requires to check their eligibility status. The basic information of the volunteer is uploaded to site and based on certain algorithms, the probable matches are shown. The user (CRO) gives the final call viewing the photographs- whether the volunteer under check is the same on the net. If not, he/she is selected and further locked by the CRO along with the new lock-in period.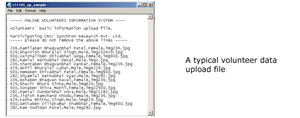
Data uploading options
OVIS offers two main approaches checking the cross participation: The ECOS (Eligibility Check On the Spot) and Volunteer List File. The ECOS approach is normally used during the screening eligibility checking where the volunteer details are directly entered on the OVIS web page. The Volunteer List File is for pre dosing eligibility check where a screened batch of volunteers are to be uploaded as well as to be blocked for certain days.Benefits of OVISTM
The exclusion can be beneficial in many ways:- Preferred by Ethics Committees as the volunteers' re-eligibility is controlled
- Financial benefits - as screening is not carried out for the ineligible volunteers trying to cross participate
- Preferred by Sponsors
OVIS and GCP
While developing OVIS, various sources were referred to ensure that the basic approach and the algorithm are in accordance with GCP. A Volunteer's photograph is used as the key information for tracing the cross participation. This might create an initial doubt about the issue of confidentiality of a volunteer's identity. After careful scrutiny of the standard sources for GCP, this cross participation platform has been created.ICMR, ICH and WMA Guidelines
OVIS offers full accordance to the below standard documents guidelines for GCP in the case of human subjects:- ICH Harmonised Tripartite Guidelines
- ICMR guideline "Ethical Guidelines for Biomedical Research on Human Subjects"
- World Medical Association Declaration: Ethical Principles for Medical Research involving Human Subjects We present here the summery of the guidelines listed in the above references eliminating the redundancy. Further we also explain its relevance with OVIS.
- Subject's identity information will not be made publicly available.
OVIS is not available to general public. The access is only given to the participating CRO. Each of them is assigned a username and password as well as a hardware key to open the service page. - Subject's identity information to be disclosed only to
authorized individuals.
Volunteers disclose their personal information to the CROs while enrolling to a trial. OVIS information is shared to these authorized CROs where the volunteers have already been and have disclosed the identity. - The rights, safety, and well-being of the trial subjects are the most important consideration.
The basic aim of OVIS is to ensure that a volunteer is not overdosed as well as the blood sampling is not carried out before 90 days (or other specified). - Volunteers' confidentiality be disclosed only after valid scientific and legal reasons. We do not disclose the names of the volunteers. We show volunteer's image (very small size sufficient to view and not for printing) to authorized CROs only for the above 'safety, well-being' of the volunteer, to check cross participation.
- To ensure the confidentiality of the subject identity should be coded. A volunteer is know by an OVIS-Id (OVIS identification no.) for any further reference.
- The List of the subjects enrolled in the trial should be kept in a confidential manner and for agreed upon time.
The site is protected as explained above. Further the volunteer information is removed as soon as they become re-eligible. (as their 90 days or other are over). - In the research on human beings each potential subject must be adequately informed of the aims, methods, potential
risks of the study, the discomfort it may entail and other details.
Each volunteer signs a consent letter where all the details of the project are supplied. The use of OVIS can also be added as the tool which will be used by the CRO. - Each participating CRO is issued a password for accessing the web based OVIS service.
- The entire data on OVIS can not be viewed by any CRO.
- No bulk download facility provided on OVIS.
- Separate upload folders provided for each participating CRO.
- The edit feature is offered to the originator of the volunteer entry. In other word a CRO can change the volunteer data only if it was uploaded by that same CRO.
- A volunteer entry can be changed only within certain hours of its generation. This is to prevent any possible mal-practice.
- Hardware lock protection to control the access to the system from limited computers.
- Software Applications Design and Development
- Software Products Development with Management tools
- Client Specific Application Development
- Web Applications and Website Development
- Data Processing and Application Protection
- IT Consulting and Application Service Provider
A CRO's Concern: Volunteer data confidentiality and protection
OVIS offers features to maintain the confidentiality and accuracy of the data uploaded by a CRO as well as the of the CRO itself :The following features / rules are implemented in OVIS:

Online Volunteers Information System - OVISTM
Web based System for Clinical Trial Organisations to track Volunteers Cross Participation(Conceptualised and developed by Inforcom Technologies)


THE ISSUE :
Contract Research Organisations (CRO) or Clinical Trial based Organisations enroll volunteers for studies. These volunteers undergo screening and further the dosing cycles. At the end of the project, they are advised not to re-participate in such project for 3 months or other specified. This time period is to ensure sufficient blood regeneration and drug wash-out. Volunteers ignore this re-eligibility locking days and get enrolled at other such organisations to earn more. This cross participation can be a health hazard to the volunteer as well a potential case of erroneous results in the studies. Inforcom Technologies is an IT consulting firm with software services and products in the field of Life Science and other. Inforcom is offering a web based application service to CROs to track such participants who hop from one CRO to another.Activities at a CRO
Volunteers approach CROs and enroll themselves for a clinical trail project. The CROs collect data of the volunteers in 'Volunteers & Project Management System - VPMS' (a clinical trial management application developed by Inforcom Technologies) or any other such tools. The participating CROs require to upload certain sharable data to OVIS - the web based system developed by Inforcom Technologies. Along with this data the lock-in period is also stored on the web. This data is the information for other Organisations to cross check the volunteers with.
The Main Features of OVIS
- 24X7 access on Internet
- Biometrix Matching
- On the spot eligibility results
- Backup check by name and photo
- Individual statistical reports
- Audit trails for work review & monitoring
- Data edit locks against tempering
- Auto Check feature for quick file processing
- Password Protection
- Validated
The Eligibility check on OVIS
As a volunteer re-approaches a CRO for screening or dosing, the organisation requires to check their eligibility status. The basic information of the volunteer is uploaded to site and based on certain algorithms, the probable matches are shown. The user (CRO) gives the final call viewing the photographs- whether the volunteer under check is the same on the net. If not, he/she is selected and further locked by the CRO along with the new lock-in period.
Data uploading options
OVIS offers two main approaches checking the cross participation: The ECOS (Eligibility Check On the Spot) and Volunteer List File. The ECOS approach is normally used during the screening eligibility checking where the volunteer details are directly entered on the OVIS web page. The Volunteer List File is for pre dosing eligibility check where a screened batch of volunteers are to be uploaded as well as to be blocked for certain days.Benefits of OVISTM
The exclusion can be beneficial in many ways:- Preferred by Ethics Committees as the volunteers' re-eligibility is controlled
- Financial benefits - as screening is not carried out for the ineligible volunteers trying to cross participate
- Preferred by Sponsors
OVIS and GCP
While developing OVIS, various sources were referred to ensure that the basic approach and the algorithm are in accordance with GCP. A Volunteer's photograph is used as the key information for tracing the cross participation. This might create an initial doubt about the issue of confidentiality of a volunteer's identity. After careful scrutiny of the standard sources for GCP, this cross participation platform has been created.ICMR, ICH and WMA Guidelines
OVIS offers full accordance to the below standard documents guidelines for GCP in the case of human subjects:- ICH Harmonised Tripartite Guidelines
- ICMR guideline "Ethical Guidelines for Biomedical Research on Human Subjects"
- World Medical Association Declaration: Ethical Principles for Medical Research involving Human Subjects We present here the summery of the guidelines listed in the above references eliminating the redundancy. Further we also explain its relevance with OVIS.
- Subject's identity information will not be made publicly available.
OVIS is not available to general public. The access is only given to the participating CRO. Each of them is assigned a username and password as well as a hardware key to open the service page. - Subject's identity information to be disclosed only to
authorized individuals.
Volunteers disclose their personal information to the CROs while enrolling to a trial. OVIS information is shared to these authorized CROs where the volunteers have already been and have disclosed the identity. - The rights, safety, and well-being of the trial subjects are the most important consideration.
The basic aim of OVIS is to ensure that a volunteer is not overdosed as well as the blood sampling is not carried out before 90 days (or other specified). - Volunteers' confidentiality be disclosed only after valid scientific and legal reasons. We do not disclose the names of the volunteers. We show volunteer's image (very small size sufficient to view and not for printing) to authorized CROs only for the above 'safety, well-being' of the volunteer, to check cross participation.
- To ensure the confidentiality of the subject identity should be coded. A volunteer is know by an OVIS-Id (OVIS identification no.) for any further reference.
- The List of the subjects enrolled in the trial should be kept in a confidential manner and for agreed upon time.
The site is protected as explained above. Further the volunteer information is removed as soon as they become re-eligible. (as their 90 days or other are over). - In the research on human beings each potential subject must be adequately informed of the aims, methods, potential
risks of the study, the discomfort it may entail and other details.
Each volunteer signs a consent letter where all the details of the project are supplied. The use of OVIS can also be added as the tool which will be used by the CRO. - Each participating CRO is issued a password for accessing the web based OVIS service.
- The entire data on OVIS can not be viewed by any CRO.
- No bulk download facility provided on OVIS.
- Separate upload folders provided for each participating CRO.
- The edit feature is offered to the originator of the volunteer entry. In other word a CRO can change the volunteer data only if it was uploaded by that same CRO.
- A volunteer entry can be changed only within certain hours of its generation. This is to prevent any possible mal-practice.
- Hardware lock protection to control the access to the system from limited computers.
- Software Applications Design and Development
- Software Products Development with Management tools
- Client Specific Application Development
- Web Applications and Website Development
- Data Processing and Application Protection
- IT Consulting and Application Service Provider
A CRO's Concern: Volunteer data confidentiality and protection
OVIS offers features to maintain the confidentiality and accuracy of the data uploaded by a CRO as well as the of the CRO itself :The following features / rules are implemented in OVIS:




Online Volunteers Information System - OVISTM
Web based System for Clinical Trial Organisations to track Volunteers Cross Participation(Conceptualised and developed by Inforcom Technologies)


THE ISSUE :
Contract Research Organisations (CRO) or Clinical Trial based Organisations enroll volunteers for studies. These volunteers undergo screening and further the dosing cycles. At the end of the project, they are advised not to re-participate in such project for 3 months or other specified. This time period is to ensure sufficient blood regeneration and drug wash-out. Volunteers ignore this re-eligibility locking days and get enrolled at other such organisations to earn more. This cross participation can be a health hazard to the volunteer as well a potential case of erroneous results in the studies. Inforcom Technologies is an IT consulting firm with software services and products in the field of Life Science and other. Inforcom is offering a web based application service to CROs to track such participants who hop from one CRO to another.Activities at a CRO
Volunteers approach CROs and enroll themselves for a clinical trail project. The CROs collect data of the volunteers in 'Volunteers & Project Management System - VPMS' (a clinical trial management application developed by Inforcom Technologies) or any other such tools. The participating CROs require to upload certain sharable data to OVIS - the web based system developed by Inforcom Technologies. Along with this data the lock-in period is also stored on the web. This data is the information for other Organisations to cross check the volunteers with.
The Main Features of OVIS
- 24X7 access on Internet
- Biometrix Matching
- On the spot eligibility results
- Backup check by name and photo
- Individual statistical reports
- Audit trails for work review & monitoring
- Data edit locks against tempering
- Auto Check feature for quick file processing
- Password Protection
- Validated
The Eligibility check on OVIS
As a volunteer re-approaches a CRO for screening or dosing, the organisation requires to check their eligibility status. The basic information of the volunteer is uploaded to site and based on certain algorithms, the probable matches are shown. The user (CRO) gives the final call viewing the photographs- whether the volunteer under check is the same on the net. If not, he/she is selected and further locked by the CRO along with the new lock-in period.
Data uploading options
OVIS offers two main approaches checking the cross participation: The ECOS (Eligibility Check On the Spot) and Volunteer List File. The ECOS approach is normally used during the screening eligibility checking where the volunteer details are directly entered on the OVIS web page. The Volunteer List File is for pre dosing eligibility check where a screened batch of volunteers are to be uploaded as well as to be blocked for certain days.Benefits of OVISTM
The exclusion can be beneficial in many ways:- Preferred by Ethics Committees as the volunteers' re-eligibility is controlled
- Financial benefits - as screening is not carried out for the ineligible volunteers trying to cross participate
- Preferred by Sponsors
OVIS and GCP
While developing OVIS, various sources were referred to ensure that the basic approach and the algorithm are in accordance with GCP. A Volunteer's photograph is used as the key information for tracing the cross participation. This might create an initial doubt about the issue of confidentiality of a volunteer's identity. After careful scrutiny of the standard sources for GCP, this cross participation platform has been created.ICMR, ICH and WMA Guidelines
OVIS offers full accordance to the below standard documents guidelines for GCP in the case of human subjects:- ICH Harmonised Tripartite Guidelines
- ICMR guideline "Ethical Guidelines for Biomedical Research on Human Subjects"
- World Medical Association Declaration: Ethical Principles for Medical Research involving Human Subjects We present here the summery of the guidelines listed in the above references eliminating the redundancy. Further we also explain its relevance with OVIS.
- Subject's identity information will not be made publicly available.
OVIS is not available to general public. The access is only given to the participating CRO. Each of them is assigned a username and password as well as a hardware key to open the service page. - Subject's identity information to be disclosed only to
authorized individuals.
Volunteers disclose their personal information to the CROs while enrolling to a trial. OVIS information is shared to these authorized CROs where the volunteers have already been and have disclosed the identity. - The rights, safety, and well-being of the trial subjects are the most important consideration.
The basic aim of OVIS is to ensure that a volunteer is not overdosed as well as the blood sampling is not carried out before 90 days (or other specified). - Volunteers' confidentiality be disclosed only after valid scientific and legal reasons. We do not disclose the names of the volunteers. We show volunteer's image (very small size sufficient to view and not for printing) to authorized CROs only for the above 'safety, well-being' of the volunteer, to check cross participation.
- To ensure the confidentiality of the subject identity should be coded. A volunteer is know by an OVIS-Id (OVIS identification no.) for any further reference.
- The List of the subjects enrolled in the trial should be kept in a confidential manner and for agreed upon time.
The site is protected as explained above. Further the volunteer information is removed as soon as they become re-eligible. (as their 90 days or other are over). - In the research on human beings each potential subject must be adequately informed of the aims, methods, potential
risks of the study, the discomfort it may entail and other details.
Each volunteer signs a consent letter where all the details of the project are supplied. The use of OVIS can also be added as the tool which will be used by the CRO. - Each participating CRO is issued a password for accessing the web based OVIS service.
- The entire data on OVIS can not be viewed by any CRO.
- No bulk download facility provided on OVIS.
- Separate upload folders provided for each participating CRO.
- The edit feature is offered to the originator of the volunteer entry. In other word a CRO can change the volunteer data only if it was uploaded by that same CRO.
- A volunteer entry can be changed only within certain hours of its generation. This is to prevent any possible mal-practice.
- Hardware lock protection to control the access to the system from limited computers.
- Software Applications Design and Development
- Software Products Development with Management tools
- Client Specific Application Development
- Web Applications and Website Development
- Data Processing and Application Protection
- IT Consulting and Application Service Provider
A CRO's Concern: Volunteer data confidentiality and protection
OVIS offers features to maintain the confidentiality and accuracy of the data uploaded by a CRO as well as the of the CRO itself :The following features / rules are implemented in OVIS:










