Data migration of CRO Volunteers data at pune CRO
![]()
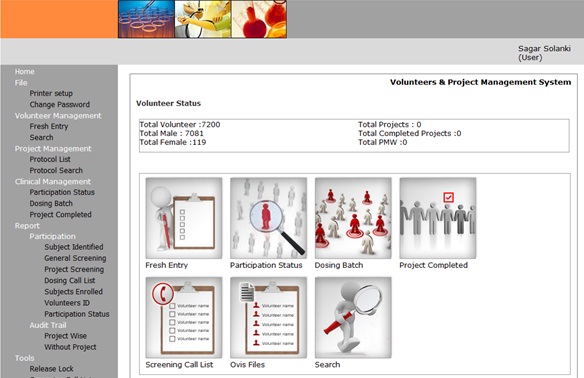
Inforcom offers VPMS - a type of Clinical Trial management System (CTMS) System. VPMS has features for the management of Volunteers and Project for CROs. The healthy volunteers study (BA / BE) requires CFR11 compliant database application. This is to manage the Registration of volunteers as well as the Clinical process. The recent project at a CRO in Pune, India, requires migration of volunteers’ demographic data. The data required integrity check as well as addition Photographs and Identity Proof scans. The information of city, state, food habits, status etc. required master tables based numeric keys. Inforcom has completed the migration of volunteers’ data for CRO from one CTMS to other. The same data needed validation. Inforcom generated the scripts and the queries for its CFR11 compliant validation. This is to prove that the data migrated has integrity, accuracy and format compliance as per the standard. Further the application VPSM will also be validated as per CFR11 requirements. VPMS is operational at 8 other locations across India. Each are validated and audited by International and Indian audit agencies as well as the sponsors. The project at the Pune CRO involves the migration of the volunteers data. Over 7000 volunteers data is to be migrated to VPMS from the existing old system. The personal, demographics data along with the and photos will be transferred to the new system. The new system directly can operate on the new data. The BA/BE studies can further be carried on these older volunteers. The data migrated will be validated with special script for its consistency and accuracy as per the entry rules.
